VOC Test Methodology
Volatile organic compound (VOC) definitions, standards and methodologies vary in different regions of the world and continue to evolve. The United States uses the EPA Method 24 oven volatility test (ASTM D2369) as a measure of VOC.1,2 ASTM D6886 is another VOC test used for some regulatory focuses and is based on a GC assay of VOC components. The data from these tests are used by paint manufacturers to assure that their paints meet the regulatory limits set for the type of paint tested in each region in the United States.
The European Union also uses a GC method (ISO 11890–2), which is specified in EU Directive 2004/42/CE to determine the relative volatilities of compounds; a VOC by this test is any compound that elutes prior to a marker compound with a boiling point of less than 250 °C. However, a 280 °C marker is now being considered as well as a “more practical test” using the ISO 16000-6 chamber method.3-6 The chamber method is specified to be a part of the German AgBB specification, but this type of method is also referred to in USGB LEED v4 as part of the testing for their certification. All of these measures relate to the actual volatility of the material used as a coalescent.
Asia tends to use the EU criterion of a 250 °C boiling point at atmospheric pressure. Again, it differs in various regions throughout that part of the world. The use of a higher boiling point marker is also of interest; in this case the boiling point cut off is 280 °C at atmospheric pressure.
All of these tests and criteria are applied to regulatory limits in one way or another. Of course, the goal is to reduce emissions of compounds deemed to be an issue environmentally and/or that contribute to poor indoor air quality. It would be virtually impossible to present all of the regulatory rules and requirements, especially on a global basis; however, the trends certainly favor reduced VOC.
The purpose of this article is to present information on volatility (VOC) measured in different ways. To illustrate the differences in results between methodologies, this study will compare a high-VOC (using the U.S. definition) conventional commercial coalescent and a commercial coalescent with low VOC to newer dibenzoate blends and monobenzoates.
Experimental
As stated above, the focus of this article is to contrast higher-VOC coalescents to the newer, lower-VOC coalescents. On that basis, examples of different classes of coalescent (chemical and VOC type) were included. Specifically, the following coalescents will be considered:
- 2,2,4-Trimethyl-1,3-pentanediol monoisobutyrate, the commercial high-VOC reference, abbreviated TMPDMIB;
- Triethylene glycol di-2-ethylhexanoate, the commercial lower-VOC reference, abbreviated TEGDO;
- K-Flex 975P – Blend of dipropylene glycol/diethylene glycol/propylene glycol dibenzoates, abbreviated 975P;
- K-Flex 850S – Blend of dipropylene glycol/diethylene glycol dibenzoates, abbreviated 850S;
- K-Flex 500P – Lower-VOC blend of dipropylene glycol/diethylene glycol dibenzoates, abbreviated 500P;
- Experimental 3-phenyl propyl benzoate, abbreviated 613;
- Experimental grade of benzyl benzoate, abbreviated BOB.
The following tests were run on the above coalescents:
- Boiling point;
- Vapor pressure;
- Flash point;
- Thermogravimetric analyzer (TGA), isothermal at 110 °C;
- ASTM D2369;
- ASTM D6886;
- Modified ASTM D6886;
- ISO 11860-2;
- ISO 16000-6 (on neat coalescents only).
The exact test methods are covered in the Appendix to this article. All of these tests give a good picture of the volatility or volatile content by different definitions. The point of this work is to illustrate ways to reduce paint VOC to pass the required regulations and ways to formulate to reduce VOCs while improving paint performance.
Discussion
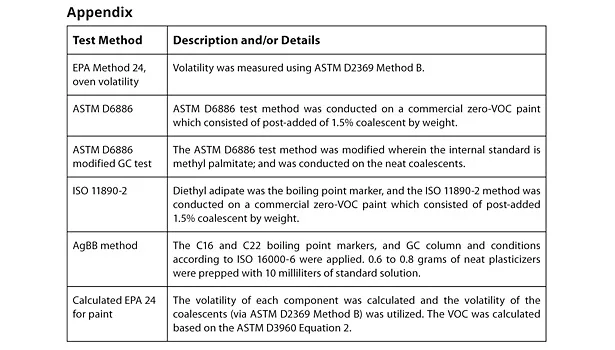
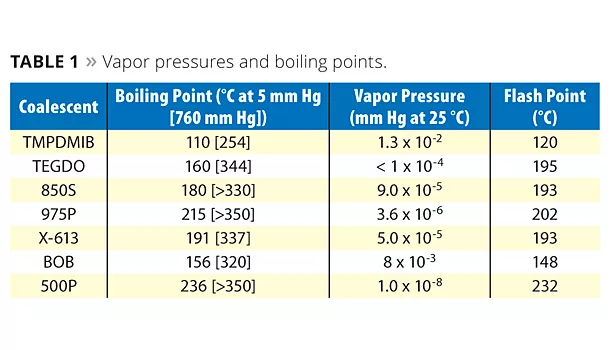
Physical data on the evaluated coalescents are listed in Table 1; Figure 1 illustrates the coalescent vapor pressures. The boiling point data indicate that both the mono and dibenzoates are very low in volatility compared to TMPDMIB; all are above 250 °C at atmospheric pressure and, therefore, all are zero VOC by the EU VOC definition. The vapor pressure and flash point data are also indicative of the low volatility of the benzoates in comparison to TMBDMIB. This data has implications for the chamber testing, which may be a test for paint in the future in both the EU and United States. The lower the vapor pressure of the coalescent, the better the expected volatility performance.
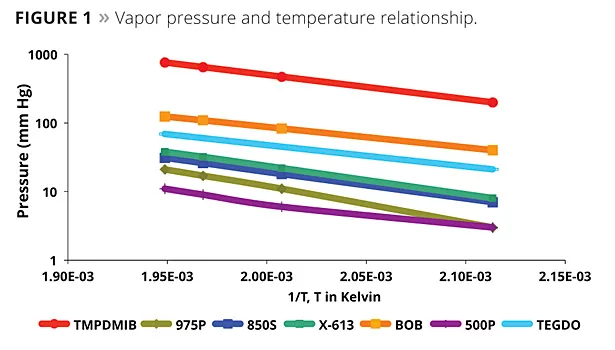
As there continues to be a drive toward lower VOCs, it is important to minimize all contributions to VOC. Even the VOC of a raw material used at a relativity low level can be significant. Oven tests are a standard method to compare the VOC contribution of volatile and semi-volatile components. Figure 2 illustrates the volatility of the coalescents of this study by the EPA 24 ASTM D2369 test. By this test, TMPDMIB is 100% volatile. While the monobenzoates are more volatile than the dibenzoate blend, both are significantly lower in volatility than TMPDMIB.
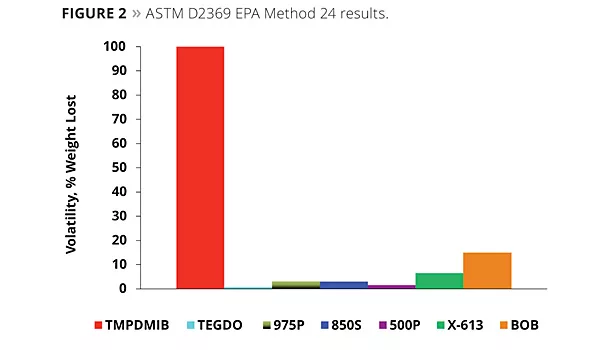
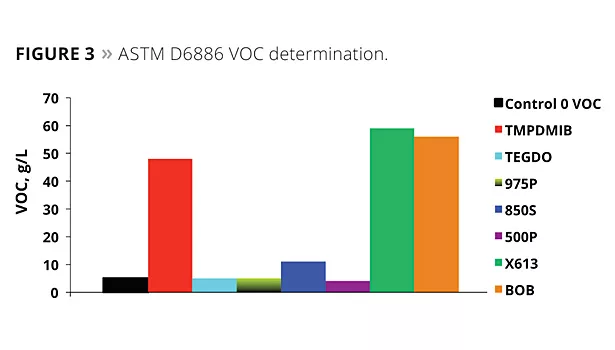
In addition to this test, the use of ASTM D6886 (GC method) is becoming more important. A commercial zero-VOC paint was used to determine the VOC by ASTM D6886, with 1.5% by weight of each of the coalescents of the study added to the paint; the ASTM D6886 GC method was then utilized for the analysis. Figure 3 illustrates the results of the testing. Virtually all of the TMPDMIB was found as VOC by this method. Also, both the 613 and BOB appeared to be 100% volatile by this method, while at the same time the D2369 test indicated that both were much lower in VOC. This highlights some of the limitations when using a GC test method, where a compound’s affinity to the GC column plays a role in addition to its relative volatility. The dibenzoates’ VOCs determined by this method were low.
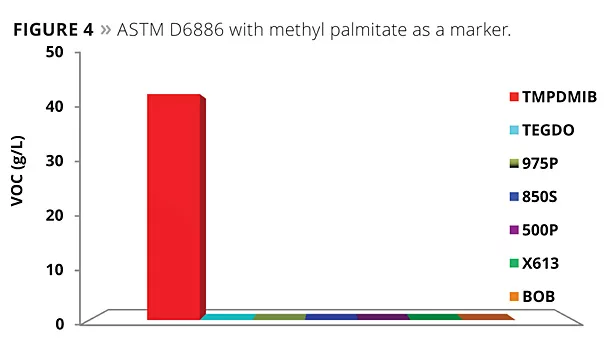
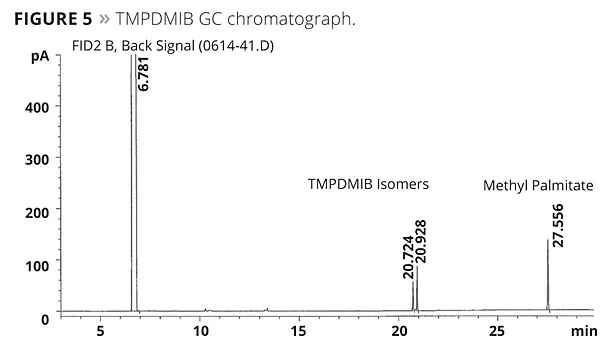
A modified ASTM D6886 test was also considered using methyl palmitate as a marker. The methyl palmitate is a very high boiler (about 323 °C at atmospheric pressure). South Coast Air Quality Management District is considering modifying their Method 313 through the use of this marker. The results of this testing are illustrated in Figure 4. All of the mono- and dibenzoates, as well as TEGDO, have a boiling point above this marker, while TMPDMIB does not. A typical GC chromatogram is shown in Figure 5.
ISO 11890-2 VOC
The EU defines a VOC as a substance with a boiling point less than 250 °C at atmospheric pressure. The ISO 11890-2 test method is used to prove that the compound being tested has a boiling point greater than the marker at 250 °C. All of the coalescents of this evaluation were found to be zero VOC using this test definition.
Chamber Test
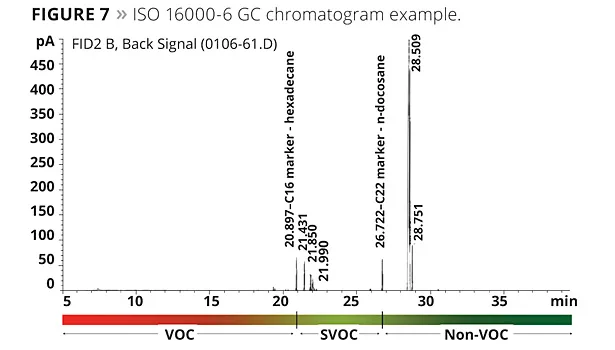
Organizations such as the U.S. Green Building LEED v4 describe the requirements for VOCs in various applications using a chamber method, as does the AgBB in Germany. The method they call for is based on the determination of the amount of VOCs released from an article over a period of a month. Based on the data obtained, the article being tested either passes or fails. Unlike the EPA 24 method or the EU boiling point criterion, the chamber method defines VOCs by references to boiling point markers. After the exposure in a chamber for specified durations, the VOCs that were trapped are then purged to a GC. In the EU, compounds detected below a C16 marker are considered to be VOCs. Compounds detected between the C16 and a C22 marker are considered to be SVOCs (semi-volatile); above the C22 marker they are considered non-VOC, even though these compounds will have some vapor pressure at the test temperature. The method also specifies alkanes as reference compounds and uses a relatively low-polarity GC column. This is not really the best approach since the compounds of interest tend to be more polar. The trap and purge technique may also have some of the same issues.
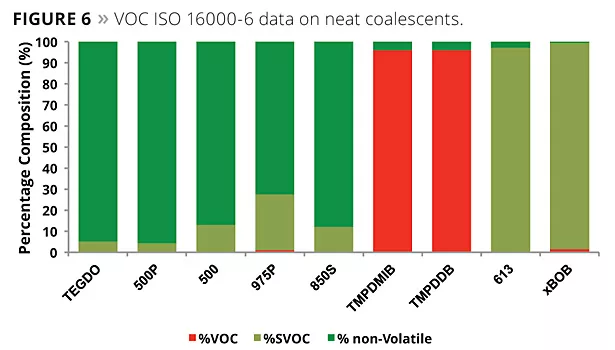
The chamber method to test paint for volatiles is meant to be used to determine the VOC for applied coatings. However, as is often performed in the United States, we ran the GC portion of the ISO 16000-6 with neat plasticizer instead of the residual from a trap and purge. It is well known that the loading in most paints will be between 1 to 3% by weight, and the matrix of the paint will also have an effect on the results of what emits during the testing. Nevertheless, we did perform testing on the neat coalescents. Figure 6 illustrates the results of this testing, and Figure 7 illustrates a GC chromatogram from the testing.
Calculated EPA 24
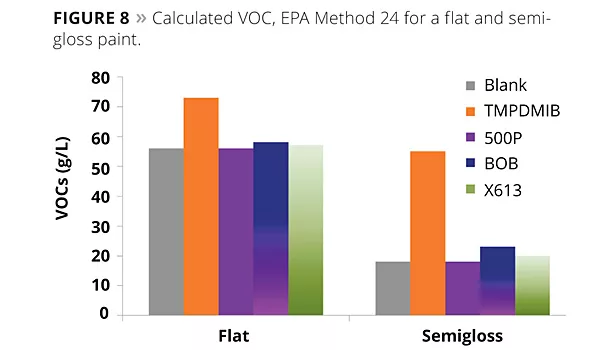
As mentioned above, it is common in the United States to calculate VOCs based on the VOCs of the neat raw materials used. This is actually incorrect. Because the matrix, as well as the compatibility of the polymer with the coalescents, will have an effect on what actually volatilizes from the coating, the calculated results tend to overestimate the VOC content. However, EPA Method 24 is difficult to use due to method errors when the actual full scheme is performed, especially for paints with low VOC contents. Figure 8 illustrates the calculated VOC of a paint based on the ASTM D2369 results of the neat components. Note that all of the paints with benzoates have lower VOCs than the traditional coalescent, TMPDMIB.
Summary
The standards for VOCs continue to evolve, and the test methodologies continue to change as the regulations for VOCs tighten. Today, a number of new low-VOC coalescents are available to the formulator. Previously published studies indicate that the new dibenzoate blend grades developed for the coatings industry perform as well as or better than TMPDMIB and TEGDO in both interior and exterior applications – excellent scrub resistance and gloss with no sacrifice in essentially all relevant performance in interior paint, and excellent performance in exterior testing, as no issues were determined on the fence.7, 8
The VOC results for a given material may vary widely depending upon test methodology. No matter which method is used to describe VOC, all of the low-VOC coalescents are indeed very low in volatility compared to the TMPDMIB control. All benzoate coalescents would contribute very little to the VOC of a coating, especially at the low level of use. When compatible, the coalescent becomes part of the binder. Specifically, the VOCs of 850S and 975P are very low to allow for better performing paints. As time goes by, there will be more need for low-VOC coalescents to allow the formulation of zero- or near-zero-VOC paints with improved performance overall.
References
1 EPA 24 Test Method, 40 CFR Part 59.
2 ASTM D-2369, Oven Volatility Method, part of EPA Method 24.
3 Reinhard, O. VOC emissions -new standards and regulations in Europe, and news from LEED, European Coating Congress, April, 2013.
4 Gernon, M.D.; Buyse, K.; Jones, D. Understanding the relative volatility of materials: A comparison of thermal analysis, GC analysis and chamber test techniques, European Coating Congress, April, 2013.
5 Krieger, S.; Reinheimer, K.; Petri, H. Overview of regulations and test methods within Europe to determine emissions from interior paints and new test results based on model paint recipes, European Coating Congress, April, 2013.
6 Testa,Carlos, Taylor, Louise, Taube, Ralf, “Meeting Current and Future Legislations with Low Emission Coalescents”, European Coating Congress, April, 2013
7 Arendt, W.; McBride, E.; Conner, M. Continuation of the Innovation of Benzoate Technology for Coatings applications, Proceedings of the 41th Waterborne, Higher-Solids and Coatings Symposium, New Orleans, LA, 2013.
8 Arendt, W.; McBride, E.; Conners, M. Innovation in Benzoate Technology for Coatings Applications, American Coatings Show and Symposium preprints, April 2014.
Acknowledgements
The authors would like to thank Emerald Performance Materials, LLC and Emerald Kalama Chemical for permission to publish and present this paper. We would also like to thank Debbie Davidson, Ian Query, Sarah Strother and Kate Williamson.
Disclaimer
The information contained herein is believed to be reliable; however it is based upon laboratory work with small scale equipment and does not necessarily indicate end-product performance. Because of variations in methods, conditions and equipment used commercially in processing these materials, Emerald makes no representations, warranties or guarantees, express or implied, as to the suitability of the products for particular applications, including those disclosed, or the results to be obtained. Full-scale testing and end-product performance are the responsibility of the user. Emerald Performance Materials shall not be liable for and the customer assumes all risk and liability for use and handling of any materials beyond Emerald’s direct control. Nothing contained herein is to be considered as permission, recommendation nor as inducement to practice any patented invention without permission of the patent owner.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!






