Polyaspartic DTM and Two-Coat Systems
Offer High Productivity and Performance







During the 1990s and early 2000s, increased competition from emerging markets drove many large manufacturing facilities to outsource production. Recently, we have witnessed an uptick in domestic manufacturing (reshoring) due to increases in both international shipping costs and in domestic manufacturing productivity driven by trends such as design-build, robotics, Lean Six Sigma analysis, and Value Stream Mapping. These tools have helped identify methods to increase productivity gains and cost-saving practices in painting operations as well. These include utilizing automated blasting equipment to reduce surface preparation time, plural-component sprayers to reduce waste and VOCs, robotic painting to increase efficiency and quality control, and painting structural steel in fabrication shops prior to shipping to the construction site.
Over the past 10 years, industrial maintenance coatings based on polyaspartic coatings have demonstrated proven performance and increased productivity in a variety of corrosion protection applications. This article reviews the chemistry and technical performance of polyaspartic coatings, and outlines their advantages and disadvantages versus traditional coatings.
Background
Polyaspartics make up a special class of hindered secondary amines whose reactivity can and has been custom-designed for desired performance in specific coating applications. The reactivity of these amines is heavily influenced by their steric hindrance and intra-molecular hydrogen bonding. Compared to typical secondary amines, polyaspartic chemistry allows coatings to be formulated with a sufficient pot life to allow the use of traditional application equipment. The selection of specific polyaspartic resin(s) and polyisocyanate(s) allows the formulator to optimize the pot life and dry time for specific applications. For example, shop-applied coatings can be formulated for plural-component application, which allows further improvement in productivity.
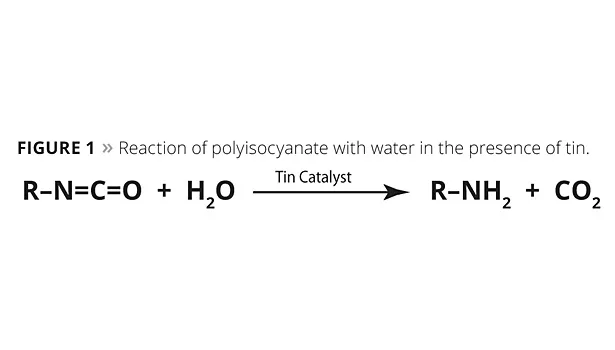
Compared to traditional polyurethane chemistry, the enhanced reactivity between the amine and the polyisocyanates in polyaspartic chemistry enables the formulator to forgo using tin catalysis, which allows the coating to be applied at higher film thicknesses than traditional polyurethane coatings. It also eliminates microblistering from the carbon dioxide gas produced by tin catalyzing the isocyanate reaction with water when polyurethane coatings are applied at high humidities (Figure 1).
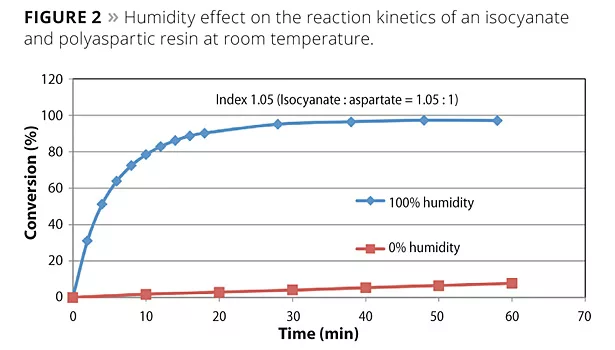
Water unaided by a tin catalyst does not react with the polyisocyanate at a competitive rate to the polyaspartic resin, dissolved water or atmospheric moisture vapor catalyzes the polyaspartic curing reaction. This enables a unique situation in which the coating cures quickly due to the coating’s surface area exposure to atmospheric moisture, yet the formulation maintains a workable pot life. Figure 2 shows the effect of humidity on the reaction conversion of a polyisocyanate and a polyaspartic resin at room temperature. At 100% humidity, the reaction approaches complete conversion in less than 20 min. Conversely, at 0% humidity, the reaction only reaches 10% of conversion in an hour.
Catalytic effects of atmospheric moisture are advantageous; however, they can cause the coating to dry at various speeds, depending on environmental conditions. Early coating formulations based on traditional polyisocyanate trimers and biurets displayed sensitivity to temperature/humidity conditions that affected not only the drying times, but also the recoat adhesion window for touch-up. In higher-temperature/humidity environments, coatings would rapidly crosslink, imparting high chemical resistance and retarding the ability of touch-up coatings to penetrate into the original coating. This can lead to inter-coating adhesive failure. Bayer has developed second-generation isocyanate crosslinkers whose reactivity is designed to overcome the aforementioned issues.1 The isocyanate crosslinker balances crosslink density, reactivity and chemical resistance, resulting in a well-rounded balance of performance properties.
While thick-film applications are advantageous when productivity is valued by the end user, these applications also create other, less-obvious advantages. The enhanced productivity has been documented in the literature2,3 and also manifests itself in reduced life cycle cost from labor cost reductions.4,5 Aside from the obvious productivity advantages, fewer coats lead to less waste, and fewer steps reduce complexity. Additional advantages of thick-film polyaspartic applications include their ability to cure at low temperatures, low-VOC formulations, compatibility with VOC-exempt solvents, and excellent edge retention and weatherability.
Due to the advantages listed above, polyaspartic technology has been successfully used in many coatings applications. Currently, the most popular application for polyaspartic formulations is for residential floor coatings, due to the technology’s rapid return-to-service and excellent weatherability characteristics. Other applications include industrial maintenance and industrial finish applications. Although these markets are more risk-averse, case studies done over many years prove the technology’s ability to perform long-term in many demanding applications and environments. Recently, three independent studies evaluated two-coat polyaspartic coatings technology. The studies were performed by NEPCOAT,6 the British Columbia Ministry of Transport7 and the Federal Highway Administration.8 All three studies found that two-coat zinc/polyaspartic systems provided performance equivalent to three-coat zinc/epoxy/urethane systems.
Case Studies
Several case studies on the service life of industrial maintenance polyaspartic technologies in a range of applications have been documented. These studies highlight the long-term performance (over eight years) of polyaspartic coatings in these applications:
- Maintenance application of a DTM polyaspartic coating on hopper railcars;9
- Shop-applied DTM polyaspartic coating on structural steel;10
- Maintenance application of a two-coat polyaspartic coating on a bridge.
In each of these applications, the ability to be applied thickly, cure rapidly and resist UV degradation were key factors in their selection.
Case Study 1 – Railcar Exteriors
In 2002, 52 hopper railcars were painted using a DTM polyaspartic coating over a SSPC SP-6 commercial blast (Figure 3). The coating was applied at 8-12 mils dry film thickness using an airless spray application unit. Each railcar was stenciled approximately 3.5 h after the polyaspartic coating was applied, and the car was moved outside to allow the coating of the next railcar. Compared to the alternative weatherable coatings system (epoxy/polyurethane), the single-coat polyaspartic coating provided a labor savings of 33% and reduced the total coating application time by one third, from 15.5 h to 10.5 h.9 Figure 4 shows that the coating’s edge retention prevented corrosion in hard-to-coat areas on the railcar’s top catwalk over 11 years of continuous service. Typically railcars are repainted after 10 years; however, these coatings far exceeded the traditional coating’s life cycle due to their inherent UV stability and thick-film performance characteristics.
Case Study 2 – Marine Corps Museum
In 2005, a new Marine Corps Museum in Quantico, VA, was rapidly constructed as a tribute to both the Marine Corps’ 230-year history and its contribution to the freedoms enjoyed by all Americans. The museum was built on a tight timeline and with strict budget constraints. The centerpiece was a soaring atrium skylight supported by structural steel beams. Due to an accelerated construction schedule targeted to meeting opening day ceremonies and the desire to avoid the cost and difficulty of painting the steel beams once they had been lifted into position, the beams were painted in the fabrication shop with a DTM polyaspartic coating. The coating’s fast cure times kept the steel moving through the fabrication shop, and its inherent light stability ensured that the coating would not fade over the years from its exposure to direct sunlight. Once fabricated and painted, the beams were carefully assembled on site and serve as the main structural support for the atrium (Figure 5).
The museum was revisited after eight years of service and, as seen in Figure 6, the appearance of the structural steel’s coating remains intact, with no fading or loss of gloss noted. Maintenance personnel reported they were extremely satisfied with the coating’s performance to date, and that only the high-touch, high-traffic handrails needed touching up since the original application.
Case Study 3 – Connecticut Bridges 1186 and 1189
In 2002 the Connecticut Department of Transportation (CT DOT) studied the performance and cost savings of using a two-coat polyaspartic (zinc epoxy/polyaspartic) versus a traditional three-coat system (zinc/epoxy/urethane). The first application of the two-coat polyaspartic technology occurred on a small bridge underpass carrying I-84 in 2002. CT DOT reevaluated this bridge in 2013 after 11 years of service (Figure 7). The coating was in excellent condition. CT DOT has no plans for additional maintenance on this bridge in the near future.
Due to the initial success of the two-coat polyaspartic system, a head-to-head evaluation of the polyaspartic technology versus the traditional three-coat system was initiated on another I-84 overpass (Figure 8). In 2002/2003 CT DOT documented the time and cost savings of the two-coat polyaspartic system over the three-coat system through rapid deployment to be approximately $6 per sq. foot, and indirect cost savings of $18 per sq. foot when including the benefits of less traffic congestion.4 The three-coat system was applied at a rate of 383 ft2 per day, while the two-coat system was applied at rate of 504 ft2 per day, representing a 31% increase in productivity.
Both the three-coat side and two-coat side of bridge 1199 were inspected in 2013 after 11 years of service. The bridge had been inspected in 2010 by a third-party inspector who reported less than 2% overall rust for both sides. The performance of the three-coat and two-coat system was compared. Overall, the traditional three-coat system was superior, with very few signs of corrosion (Figure 9). The two-coat system looked well, but did have some areas of rust due to under-application and poor surface preparation. In areas where the polyaspartic coating was properly applied, the coating’s condition remained excellent.
Conclusions
Recently, we have seen a resurgence of domestic manufacturing due to significant technology and design improvements. Design improvements in infrastructure fabrication require that every step in the construction process be as lean as possible. Polyaspartic coatings are one cost-saving solution – offering both increased productivity and reduced labor costs in tough corrosion protection applications. Polyaspartic coatings provide the long service life necessary for long-term asset protection.
References
1 Desmodur® XP 2763 brochure.
2 Fultz, B.; Corbert,W.; Best, K. “Time is Money: Improving Shop and Field Coating Throughput by Reducing Finish Coat Handling Time” SSPC Proceedings 2011.
3 Castler, B. “Rapid Deployment Technology: A New Concept for Connecticut,” International Bridge Conference, Engineers Society of Western Pennsylvania, Pittsburgh, PA, June 10, 2003.
4 Kline, E.S.; Angeloff, C.A. P.E., “Rapid Deployment: A Potential Approach to Reducing the Costs of Painting Steel Overpass Bridges over Busy Highways,” JPCL2000, 1, 79–85.
5 Helsel J. “Practical considerations for the life cycle evaluation of zinc rich coatings, galvanized steel and thermal sprayed metals for industrial structures in moderate environmental exposures” SSPC Proceedings 2007.
6 http://data.ntpep.org/Module/SSC/Search.aspx.
7 O’Donahue M., et al. “Innovative Coating Systems for Steel Bridges: A Review of Developments” JPCL 2013, 1, 34-52.
8 www.fhwa.dot.gov/publications/publicroads/06sep/06.cfm.
9 Bayer Railcar BTR.
10 Bayer Marine Corps Museum BTR, www.bayermaterialsciencenafta.com/4/MarineMuseumBTR.pdf.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!








