Polyurethane Polymers, Part 1
Hamster3d, Vetta, via Getty Images.
When superior film properties are needed, a two-component (2K) system will have superior properties compared to a one-component (1K) system. These properties include water resistance, corrosion resistance, chemical resistance, abrasion resistance, and many others.
Two of the more common 2K coatings systems are polyurethane and epoxy. In this and next month’s columns, we will concentrate on urethanes. While I will use polyurethane and urethane in this article, the reference will be to polyurethane polymers. Polyurethanes are formed by the reaction of an alcohol and an isocyanate. The polyol’s hydroxyl group reacts with the following order of speed of reaction: primary > secondary > tertiary.
In the presence of water or amines, a polyurea will form instead of a polyurethane, which will change the final film properties. The evolved CO2 will form bubbles in the film if the polyurea reaction is not complete before the coating viscosity increases, causing film defects from the trapped bubbles. Figure 1 (b and c) shows the reaction.
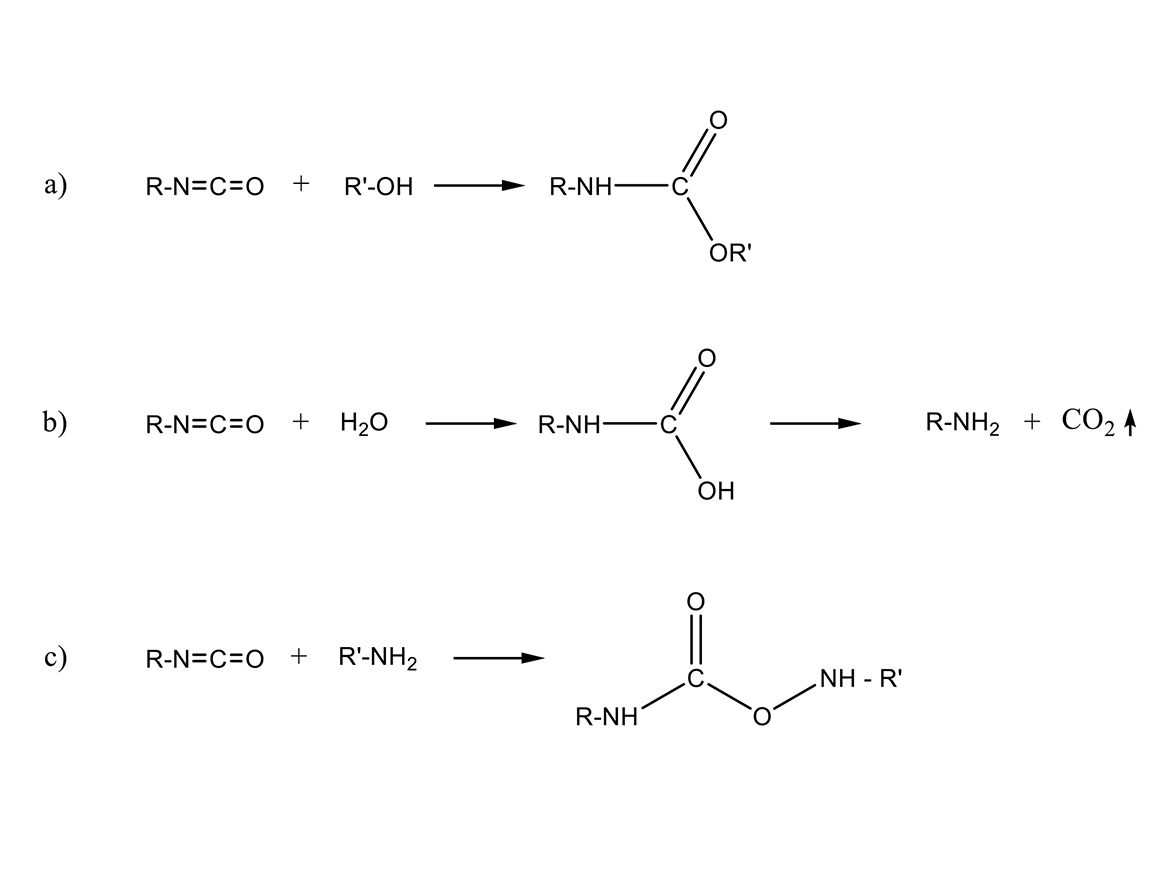
In Figure 1, there are three reactions. Figure 1(a) is the reaction of an isocyanate and a polyol, yielding a urethane. This is generally the desired reaction for coatings to avoid defects from bubbles. The advantage of urethane over urea polymers is the increased flexibility and toughness of the urethane. In the presence of water, the isocyanate will react with the water, generating an amine group and CO2 gas. Since amines react significantly faster with isocyanates than hydroxyl groups, the result will be the formation of urea linkages, instead of urethane linkages (Figure 1b and b).
Also of note is the fact that one water molecule will consume two isocyanate groups in this reaction. If we take methylene diphenyl diisocyanate (MDI), for example, 18 grams of water will consume 250 grams of MDI. It will also react with 168 grams of hexamethylene diisocyanate (HDI). When polymeric versions of these isocyanates are used, the amount of isocyanate consumed is increased at least 50%. So, a bit of water goes a long way in consuming isocyanate. When formulating urethanes, excess isocyanate is required over the normal 1:1 isocyanate to polyol stoichiometry. In the case of high-build coatings (greater than 50 mils, 1,250 microns) an excess of 3-6% isocyanate is recommended. For thinner coatings, 5% to 10% excess isocyanate is recommended. Normally, we do not talk in terms of % excess isocyanate, but rather a ratio of isocyanate to polyol, and a 5% excess is a 1.05 ratio.
Since most monomeric isocyanates have toxicity issues, polymeric isocyanates or pre-polymers are used in coatings. A pre-polymer is when a polyol is reacted with two or more isocyanates to yield an isocyanate-terminated polymeric isocyanate. The functionality (number of reactive groups per unit) of the polymeric isocyanate is based on how many diisocyanates reacted with the polyol groups.
Polymeric isocyanates are made from the reaction of two or more diisocyanate molecules, and the total number of isocyanate groups is half of the original total for each diisocyanate used. Therefore, if three diisocyanates are reacted together, you end up with a triisocyanate polymer since isocyanate groups are reacted to form the polymer.
Although there are many different isocyanates, they normally fall into two categories: aromatic or aliphatic. Figure 2 shows the structures of common isocyanates. The increase in molecular weight reduces the volatility of the polymeric isocyanate and reduces environmental, health, and safety (EHS) concerns.
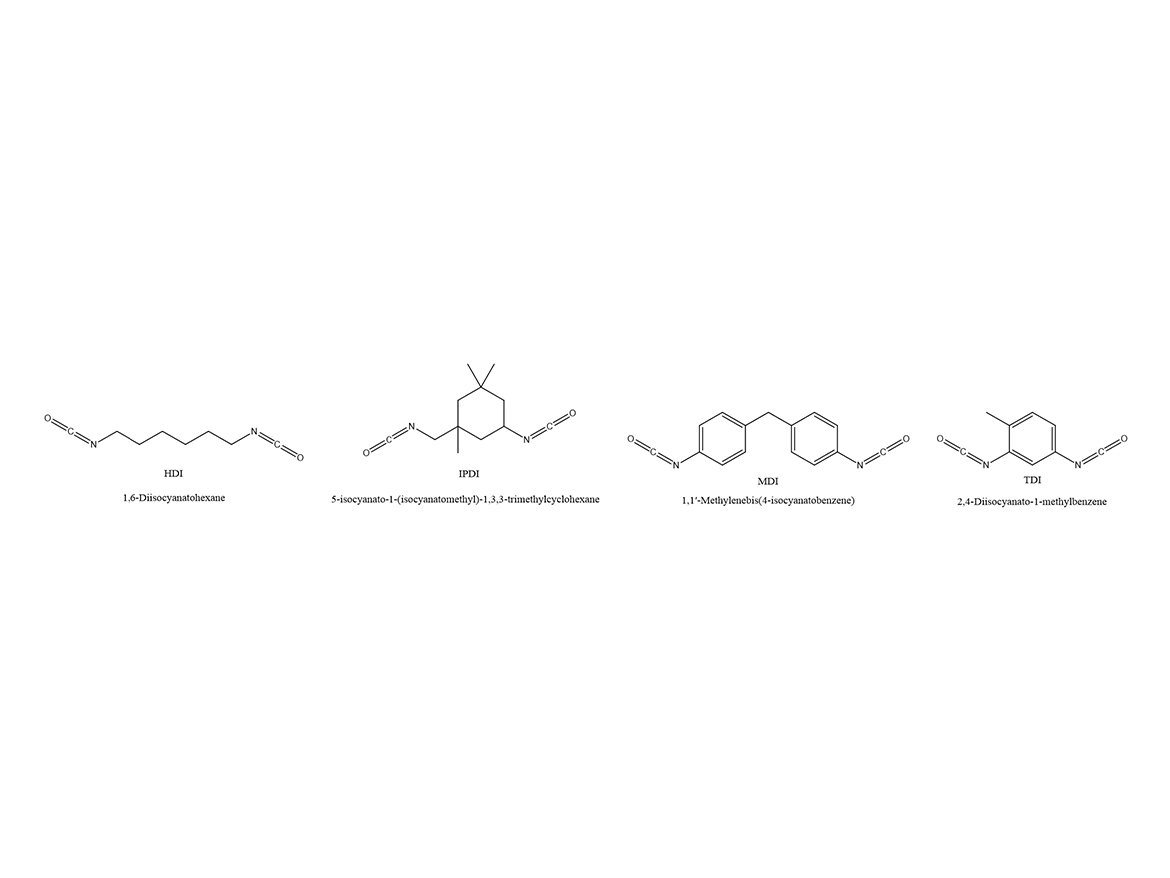
Aromatic isocyanate is where the -N=C=O group is attached to an aromatic ring. The most common aromatic isocyanates are methylene diphenyl diisocyanate (MDI) or toluene diisocyanate (TDI). Aromatic isocyanates react faster than aliphatic isocyanates, and an external catalyst is not always needed. They tend to give harder films and have better chemical resistance properties as well. However, they have very-poor UV resistance and are generally not suitable for exterior applications that have UV exposure. TDI is rarely made into the polymeric form. MDI production yields a mix of products, including monomeric and polymeric forms. Often a blend of these are sold by viscosity – 200 centipoise and 700 centipoise are the most common. Average functionality of the 200 and 700 centipoise is around 2.7 and 3, respectively.
Aliphatic isocyanates have the N-C=O attached to an aliphatic backbone. They are known for excellent weathering properties and good overall properties. They tend to have slower reaction rates with polyols and usually require a catalyst. The most common of these are hexamethylene diisocyanate (HDI) or isophorone diisocyanate (IPDI). Monomeric aliphatic isocyanates are used to make polyurethane dispersions and moisture-cure pre-polymers. Two-component coatings will be made using polymeric aliphatic isocyanates.
With the advantages of urethanes over ureas in terms of physical properties (normally superior flexibility and abrasion resistance for the same hardness), one would wonder why polyurea coatings are used. The main reason is reaction speed. Aliphatic isocyanate cure is sluggish, and the fast amine isocyanate reaction yields very-fast return to service times.
Polyols used in coatings (usually polymeric) can also have different functionality. This, combined with isocyanates having varying functionality, creates coatings with low to high three-dimensional cross-linked structures. Typically, higher functional components used in a urethane will result in greater rigidity. Higher chemical resistance properties will come from a higher crosslink density (higher proportion of the molecular weight being urethane).
Common polyols are acrylics, alkyds, polyesters, polyethers, polycarbonates, or fatty acids or alcohols from natural oils.
Polyurethanes can be made from polyols and isocyanates with contrasting glass transition temperatures, resulting in coatings with alternating hard and soft segments (not random like some polymers). This creates a polyol with the ability to be both hard and flexible, unlike most polymers that are either hard and inflexible or soft and flexible. The possible permutations of combining diverse polyols, isocyanates, and functionalities result in a spectrum of performance possibilities. The next article will cover more polyurethane topics.
All information contained herein is provided "as is" without any warranties, express or implied, and under no circumstances shall the authors or Indorama be liable for any damages of any nature whatsoever resulting from the use or reliance upon such information. Nothing contained in this publication should be construed as a license under any intellectual property right of any entity, or as a suggestion, recommendation, or authorization to take any action that would infringe any patent. The term "Indorama" is used herein for convenience only, and refers to Indorama Ventures Oxides LLC, its direct and indirect affiliates, and their employees, officers, and directors.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





