Using Simulation Tools and Physicochemical Properties to Guide the Selection of Additives, Part 4
Hansen Solubility Parameters

Davizro / iStock / Getty Images Plus, via Getty Images.
In this month’s article, we will continue using physicochemical properties to help streamline additive selection. In the previous column, we discussed using free surface energy per area. We will now move on to using Hansen solubility parameters (HSP).
One way to predict the strength of the interaction between a dispersant and a pigment is by simulating the Hansen solubility parameters of the affinic pigment groups and calculating the relative energy difference (RED) between the affinic pigment group and the pigment of interest. Table 1 presents the simulated HSP values for six different affinic pigment groups and the calculated RED for three different pigments: carbon black, PBk7; phthalocyanine blue, PB 15:3; and titanium dioxide, PW6. The values in parentheses after the pigment name illustrate the values of δD (dispersion or van der Waals forces), δP (polarity related to dipole moment), δH (hydrogen bonding), and radius. These values were determined experimentally and obtained from an HSP prediction database.
The results above indicate that affinic group D is the best one for carbon black since it has the lowest RED value, which means it would be the most similar or the one with the best affinity to the pigment of interest¹. For the blue pigment, affinic group F appears to be the highest-recommended one. For titanium dioxide, any of the affinic groups seems to be appropriate, which is expected since titanium dioxide usually requires charged groups to be dispersed, and these are not being taken into account in this simulation because only the affinic group (hydrophobic group) is being evaluated.
The use of simulated HSP parameters of the affinic group is very useful for developing new molecules, but this information is rarely available to pigment dispersion formulators since it is sensitive information considered proprietary by surfactant or dispersant producers. Therefore, to help pigment dispersion formulators better compare products, the experimental determination of HSP parameters is more useful. Table 2 compiles the HSP parameters of two surfactants derived from affinic group D. The first one, surfactant 2, was already covered in the previous article. The other one, surfactant 5, is a phosphate version of surfactant 2. The HSP parameters were determined experimentally.
Based on the experimental results, simulations were prepared to compare the affinity of these surfactants with a carbon black pigment. Table 2 also contains the RED values calculated.
Interestingly, the dispersants used present a two-sphere model, which could be explained by the very different nature of the hydrophobic and hydrophilic portions of these surfactants. As shown in Table 2, the hydrogen bonding parameter (δH) is significantly higher for both Sphere 2 values, which is probably the sphere that covers the hydrophilic portion of the surfactant — the poly(ethylene glycol) chain. The same trend is observed for RED. Sphere 1 of both dispersants presents low RED values, showing higher affinity to the pigment surface, which is expected since the adsorption of these surfactants onto the surface of the pigment is probably driven by van der Waals forces between the hydrophobic portion of the molecule and the pigment.
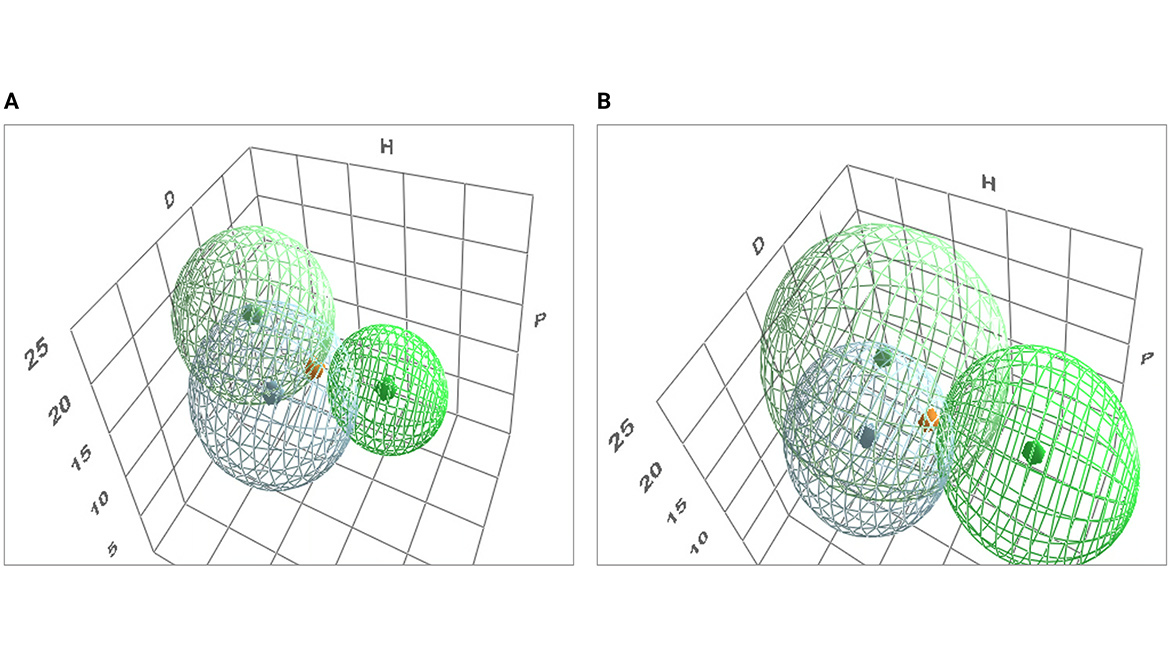
Figure 1 shows the two-sphere model for surfactants 2 and 5 and depicts the interaction with the sphere for carbon black, which was already uploaded in HSP software. The RED with the carbon black pigment for surfactant 2 is lower for both spheres when compared with surfactant 5, as seen in Table 2. This result indicates that surfactant 2 seems to have more affinity for the pigment than surfactant 5. To further investigate it, adsorption isotherms for both surfactants were prepared and will be covered in the next article.
The application results between dispersants 2 and 5 are presented in Table 3 and show efficient and similar behavior between the dispersants. Both present a surfactant actives on pigment (SOP) level of 12.0% and similar tinting power and rub-out values. Interestingly, dispersant 5, which presents higher adsorption, is also better for viscosity control after the heat-age test. This is probably due to the larger amount of dispersant on the surface of the pigment, which avoids agglomeration. Dispersant 2 also exhibits excellent performance, which is probably due to its great affinity with the pigment of interest.
Conclusions
The use of Hansen solubility parameters, obtained through both simulation and experimental studies, can be extremely useful in the process of developing new dispersing agents since affinic groups can be selected to specifically target one or more pigments of interest, creating an extremely specific dispersant or a more universal or versatile grade. On the other hand, to support formulators in selecting products, the experimental determination of HSP is a great tool to compare different technologies without the need to fully know the composition and structure of the dispersant, which in most cases is not easily available to the formulator. The use of the HSP concept and data can make product selection more assertive and require fewer experimental application tests for the final choice.
References
- van Loon, S.; Fricker, B. Using HSP to improve dispersibility of pigments and fillers. SpecialChem. https://www.specialchem.com (accessed 2025-01-17).
Acknowledgements
I will say that without Alann O. P. Bragatto, Suzy S. Alves, Beatriz Pinto, Rafael S. Dezotti, Robson Pagani, Bruno S. Dario, and Fabricio. G. Pereira (of Indorama Ventures:Indovinya Brazil), none of this would be here. Their work in this area is truely inspirational, and I have learned a lot from them. In fact they are the reason we won best paper at both the 2025 Waterborne Symposium and the 2025 Eastern Coatings Show. While I accepted the awards, they made it possible.
All information contained herein is provided "as is" without any warranties, express or implied, and under no circumstances shall the author or Indorama be liable for any damages of any nature whatsoever resulting from the use or reliance upon such information. Nothing contained in this publication should be construed as a license under any intellectual property right of any entity, or as a suggestion, recommendation, or authorization to take any action that would infringe any patent. The terms "Indorama" and “Indovinya” are used herein for convenience only, and refer to Indorama Ventures Oxides LLC, its direct and indirect affiliates, and their employees, officers, and directors.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







